Page 85 - 2019
P. 85
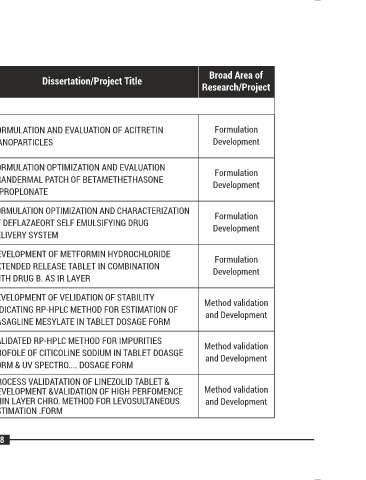
POST GRADUATE DISSERTATIONS
Sr. Enrollment Name Dissertation/Project Title Broad Area of
No. No. Name of Student Branch of supervisor Research/Project
Faculty of Pharmacy (M.Pharm.)
F F aculty of Pharmacy (M.Pharm.)
aculty of Pharmacy (M.Pharm.)
Pharma- FORMULATION AND EVALUATION OF ACITRETIN Formulation
08 17024671008 PATEL MAYUREE D. Prajapati B. G.
ceutics NANOPARTICLES Development
FORMULATION OPTIMIZATION AND EVALUATION
Pharma- Formulation
09 17024671009 PATEL NISHI M. Patel R. P. TRANDERMAL PATCH OF BETAMETHETHASONE
ceutics Development
DIPROPLONATE
FORMULATION OPTIMIZATION AND CHARACTERIZATION
Pharma- Formulation
10 17024671010 PATEL PRIYANKA S. Patel R. P. OF DEFLAZAEORT SELF EMULSIFYING DRUG
ceutics Development
DELIVERY SYSTEM
DEVELOPMENT OF METFORMIN HYDROCHLORIDE
Pharma- Formulation
11 17024671012 SOMYA VERMA Prajapati B. G. EXTENDED RELEASE TABLET IN COMBINATION
ceutics Development
WITH DRUG B. AS IR LAYER
DEVELOPMENT OF VELIDATION OF STABILITY
Quality Method validation
12 17024611001 CHAUHAN EKTA V. Patel Paresh U. INDICATING RP-HPLC METHOD FOR ESTIMATION OF
Assurance and Development
RASAGLINE MESYLATE IN TABLET DOSAGE FORM
VALIDATED RP-HPLC METHOD FOR IMPURITIES
KERALIYA Quality Method validation
13 17024611002 Patel Dipti B. PROFOLE OF CITICOLINE SODIUM IN TABLET DOASGE
SWATIBEN G. Assurance and Development
FORM & UV SPECTRO.... DOSAGE FORM
PROCESS VALIDATATION OF LINEZOLID TABLET &
LIMBACHIYA Quality DEVELOPMENT &VALIDATION OF HIGH PERFOMENCE Method validation
14 17024611003 Patel Sejal K.
DHARA R. Assurance THIN LAYER CHRO. METHOD FOR LEVOSULTANEOUS and Development
ESTIMATION .FORM
58